Why Does Copper Have a High Melting Point
0 0 Get the Free Answr app Click a picture with our app and get instant verified solutions Scan Me OR. Copper Melting Point and Boiling Point.

Pin By Matthew Venn On 11 K Villagers It Cast Element Symbols Metal
Why does Copper have a much higher melting point than Tin but a lower boiling point.

. Can we use copper wire instead of Nichrome. Copper melts at 1984 F Tin melts at 450 F Copper boils at 4644 F Tin boils at 4717 F. At a high temperature copper carbonate decomposes to carbon dioxide and copper II oxide.
Ionic bonds need more energy when u heated it up ur energizing it. Why does arsenic have a high melting point. Why does copper chloride have a high melting point.
Why does the melting point increase as you go down groups anyway. Tungsten has a high melting point and high resistivity. Covalent compounds tend to be soft and relatively flexible.
Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions. Melting Point Transition metals have high melting points due to their strong metallic bonding. CuCO 3 solid CuO solid CO 2 gas Copper Alloys.
Therefore covalent compounds have low melting and boiling point. Heat is measured in units of energy so thats why the boiling point for metals require lots. Copper has a higher melting point than zinc.
Note that these points are associated with the standard atmospheric pressure. All ionic compounds have high melting points for this reason. Boiling point of Arsenic is 614C.
Answer 1 of 10. More delocalized electrons the stronger the metallic bonds. Copper has a high melting point - basically put - is because the bond of copper atoms held together by electro statical attraction to themselves is so strong only a very high temerature can break the bond.
Pure tungsten is used in bulbs. As a result the binding energy in its crystal lattice is very high. As metals are giant lattice structures the number of electrostatic forces to be broken is extremely large and so metals have high melting and boiling points.
Melting point of Arsenic is 817C. They do not conduct electricity. As metals are giant lattice structures the number of electrostatic forces to be broken is extremely large and so metals have high melting and boiling points.
However the group 12 metals have much lower melting and boiling points since their full d sub. Does tungsten have high resistance. Melting point from nitrogen to arsenic increases from arsenic it decreases upto bismuth because down the group as the size of the element increases the tendency of elements to form three covalent bonds increases inert pair effect.
They have high melting points and boiling points because the metallic bonding in the giant structure of a metal is very strong - large amounts of energy are needed to overcome the metallic bonds. This requires more heat energy to overcome. Copper in moist air oxidizes to a dull green color and therefore is used by architects to create some interesting features on buildings.
Both boiling and melting points are due to inter-atom interactions. Melting point of Copper is 108462C. These properties are due to metallic bonding by delocalized d electrons leading to cohesion which increases with the number of shared electrons.
Answer 0 3lyosh because of its strong metallic bonds. The melting point of copper is higher than that of zinc because the d-electrons of copper are involved in metallic bonding. While copper has a charge of 1 and a bigger radius.
It has a high melting point and boiling point as much energy is needed to break the many strong covalent bonds. Boiling point of Copper is 2927C. Metallic bonding is defined by delocalization of valence electrons leaving the metal cations to be embedded in a sea of delocalized electrons.
Metals have the highest boiling and melting points because they have the strongest chemical bonds which are metallic. Light is radiated from the bulb-filaments as a drop in electrical energy of the circuit. The bonds between these two atoms are very hard to break.
Copper melts at 1085C while zinc melts at 4195C. The difference in melting points for ionic compounds can be explained by the size of the ions. It is a fact that we use tungsten for a filament.
The strongest chemical bonds require the most energy to break apart. It has a high melting point and boiling point as much energy is needed to break the many strong covalent bonds. It does not conduct electricity as there are no electrons free to move and carry charge.
It is used as a desiccant for drying air streams. In general transition metals possess a high density and high melting points and boiling points. This means that the melting point and boiling point of metals are more similar to those for ionic compounds than for covalent substances.
This means that the melting point and boiling point of metals are more similar to those for ionic compounds than for covalent substances. Solve any question of The d and f Block Elements with- Patterns of problems Was this answer helpful. Why does copper have a higher melting point than aluminium.
Covalent compounds have low melting point due to the weak van der Waals forceshus less energy is required to break the force of bonding. Copper has a curious d-shell half-filled and a half-filled 2s shell. Properties of covalent compounds.
Why copper has a high melting and boiling point. Aluminium has higher charge 3 and a smaller radius meaning that there is a higher charge density and thus stronger forces of attraction to the lattice. The melting point of copper carbonate is 200 C.
Melting Boiling Points Of Metals Science Education Matters

The Melting Points Of Metals Metal Supermarkets Uk

Melting Points Of Various Metals Metal Working Tools Metal Melting Point
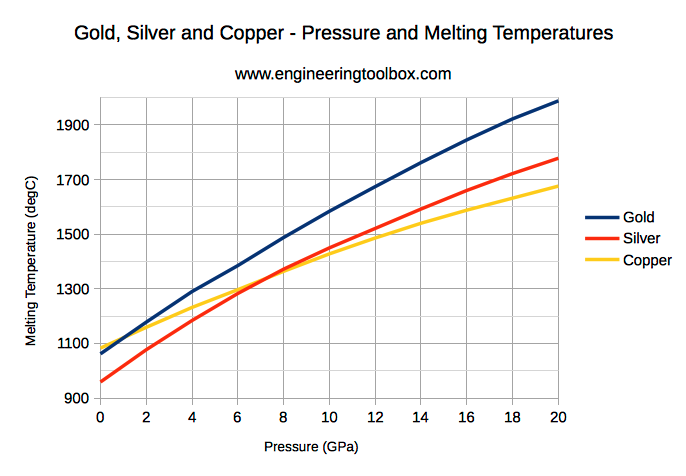
Metals And Alloys Melting Temperatures

Guide To Metal Melting Points ºf Youtube

Melting Point What Makes Some Metals Melt At Higher Temperature Chemistry Stack Exchange
17 Metals With The Highest Melting Points And Why Materials Science Engineering

Copper 29 Cu Properties And Uses Of Copper Copper Has A Melting Point Of 0 2 C Copper Has A Melting Point Of 0 2 C It Ppt Download
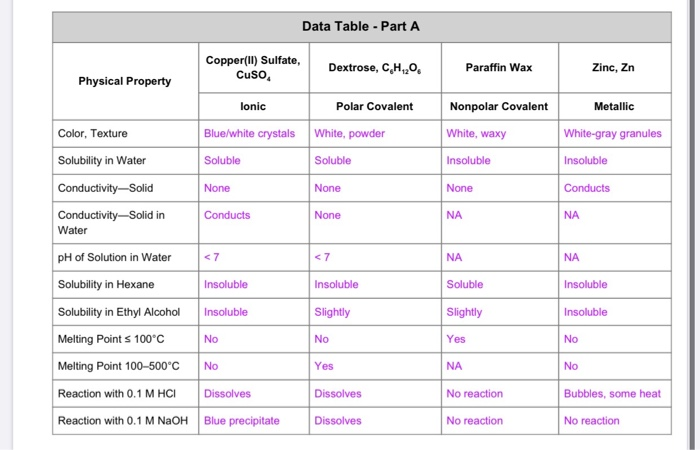
Solved 5 Sep Construct Explanations Why Are The Melting Chegg Com
What Is The Melting Point Of Pure Copper Cu And What Other Metals Are Usually Added To It For Commercial Purposes Quora
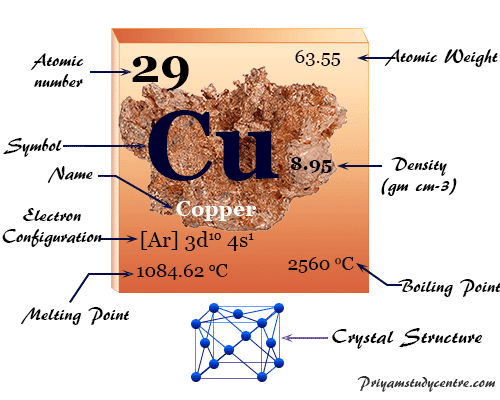
Copper Facts Symbol Properties Compounds Uses
Why Do Metals Have High Melting And Boiling Points Quora
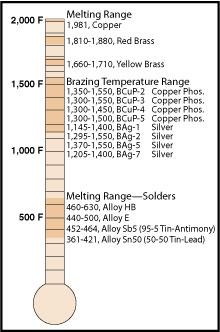
Brazing Copper And Copper Alloys

Melting And Boiling Point Copper Boiling Point Element Project

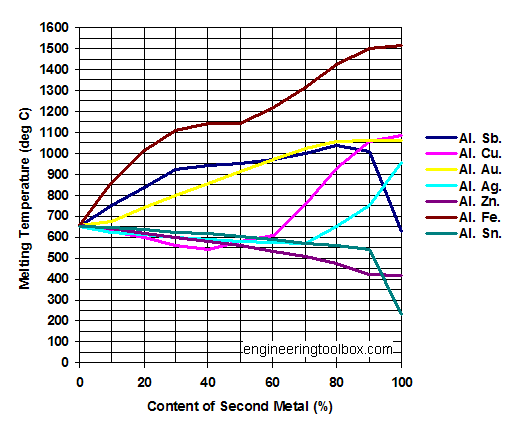
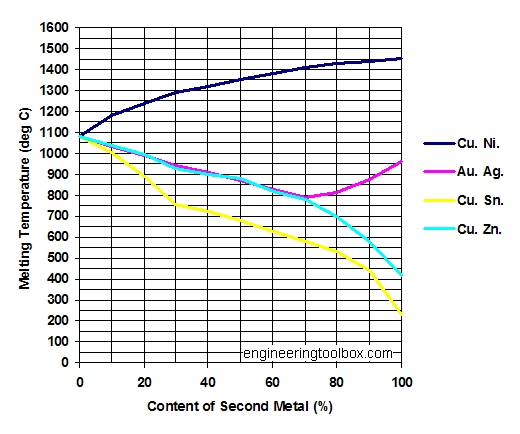
The Melting Point Of Copper Is Higher Than That Of Zinc Because
Metals With High Melting Points Matmatch
15 Metals With The Lowest Melting Point Materials Science Engineering
Comments
Post a Comment